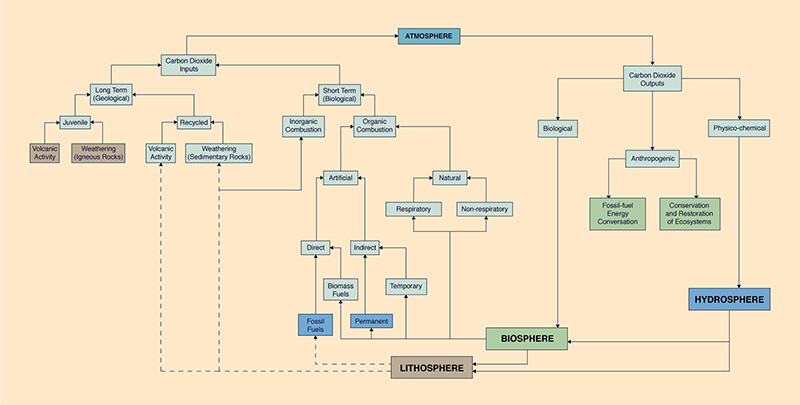
1. INTRODUCTION The issue of global climate change is upon us. The change has been broadly recognized and is now being carefully monitored (IPCC, 1996a). Initial steps are being taken in the global political and diplomatic arenas to cope with global climate change (United Nations, 1994). The issue has significant implications affecting nearly every facet of human activity, from climatology and hydrology, to economics and politics. Despite the clear signs that a global climate change is upon us, the issue remains controversial. Many people are skeptical that a problem exists, or if it does exist, that an anthropogenic cause is at the root of It. The problem may be best viewed using a systemic approach. The Earth's atmosphere is a reservoir containing several gases, some of which affect the global climate. Like any other reservoir, the atmosphere is subject to inputs, outputs, and storage. The examination of these inputs and outputs, their rates and amounts, and of the historical storage levels can lead to a better understanding of global climate change. 2. CAUSES OF CLIMATE CHANGE The atmospheric greenhouse gases function as the glass of a greenhouse, allowing solar energy to reach the Earth's surface but trapping the heat energy emitted by the Earth. Thus, these gases have a direct relation to the global mean surface air temperature. In the past two centuries, measured increases in the concentration of greenhouse gases have been accompanied by increases in global mean surface air temperaturere. The latter affects the volume of the polar icecaps and, consequently, the global mean sea level (IPCC, 1996a). Recent data shows that the 1995 global mean surface temperature was 0.4 °C above the 1961-90 average, and 0.04 ℃ above 1990, which was the previous warmest year in the record (Halpert et al., 1996). At the current concentration of 363 parts per million by volume (ppmv) (1998 data), carbon dioxide is the most significant greenhouse gas. Other greenhouse gases such as methane, nitrous oxide, and chlorofluorocarbons (CFC's) are present, albeit in smaller quantities. Whereas methane and nitrous oxide are produced by both natural and artificial processes, CFC's are entirely anthropogenic. As a greenhouse gas, methane is 20.6 times more effective than carbon dioxide; however, its concentration is only 1.72 ppmv, equivalent to 9.8 percent of the carbon dioxide. Nitrous oxide is 206 times more effective than carbon dioxide, but its concentration is 0.312 ppmv, equivalent to 17.7 percent of the carbon dioxide. CFC's are 12200 to 15600 more effective than carbon dioxide, but their concentration is only about 0.001 ppmv, equivalent to 3.83 percent of the carbon dioxide (IPCC, 1996a). The global phaseout of CFC's is well underway (IPCC, 1996a). However, research to establish the sources and sinks of methane and nitrous oxide continues. In this paper we consider only carbon dioxide, the most important greenhouse gas. We first examine inputs and outputs, and then, the historical concentration levels in the atmosphere. 3. INPUTS OF CARBON DIOXIDE TO THE ATMOSPHERE Inputs of carbon dioxide to the atmosphere are: (1) long term (geological), and (2) short term (biological or anthropogenic) (Fig. 1). The geological inputs may be either juvenile or recycled, and they can arise from volcanic eruptions or from rock weathering. Early geological inputs occurred through the degassing of the Earth as it cooled in the first one billion years of its development (Henderson-Sellers, 1983). Igneous rocks are the source of juvenile inputs, whereas sedimentary rocks produce recycled inputs. Volcanic eruptions, which have been been occurring for the past 2.8 billion years, contribute large quantities of inorganic materials to the atmosphere, among them, carbon dioxide, sulphur dioxide, and water vapor (Cattermore, 1989). Rock weathering has been occurring since the time of their formation. The weathering of rocks is a very slow process; consequently, it releases relatively small quantities of carbon dioxide to the atmosphere. The combustion of inorganic matter adds carbon dioxide to the atmosphere, but the amounts are too small to be of practical significance. On the other hand, the combustion of organic matter constitutes the main input of carbon dioxide to the atmosphere. Hereafter, we use the word combustion to refer only to organic combustion.
Combustion may be either: (1) natural, or (2) artificial. Natural combustion takes place as respiratory combustion, i.e., the respiration of animals, or as nonrespiratory combustion, i.e., the burning of vegetation, such as in naturally occurring forest fires. Artificial combustion, which is that produced by humans in the course of pursuing their livelihood,
can be either: (1) direct, or (2) indirect. Indirect artificial combustion occurs when humans act to reduce the biological or geological processes by which carbon is removed from the atmosphere. An example of indirect artificial combustion is the clearing of forests or rangelands to use the land for animal husbandry, agriculture, and other economic activities. The clearing of forests may involve only indirect combustion (e.g., logging), or both direct and indirect combustion (e.g., the extraction of firewood). Indirect artificial combustion may be temporary or permanent. Temporary indirect artificial combustion reduces the effectiveness of the carbon sink for a relatively short period (years to decades). Reducing the effectiveness of the carbon sink decreases the net biological productivity of an ecosystem. Net biological productivity is the difference between the sum of gross productivities and the sum of respirations, encompassing the entire food chain. This definition applies to the biosphere as a whole; transfers between neighbodng ecosystems would need to be accounted for in regional biochemical mass and/or energy budgets.
An example of temporary indirect artificial combustion is slash-and-burn agriculture, a practice which prevails in many primitive societies. After several years of exploitation, lands subjected to slash-and-burn are abandoned, and through secondary succession, they eventually return to the original ecosystem. Permanent indirect artificial combustion reduces the effectiveness of the carbon sink for a relatively long period (centuries to millennia). Examples of permanent indirect artificial combustion are
land use changes such as the conversion of: (1) forests to grasslands, (2) grasslands to farmlands,
(3) farmlands to urban areas, and Figure 1 shows the circulation of carbon through the ecosphere. The juvenile inputs constitute sources of fresh inorganic carbon, which are responsible for the inception of the carbon cycle. Recycling through the biosphere (solid lines) is comparatively fast, whereas recycling through the lithosphere (dashed lines) is characteristically slow. Two inputs are nonrecyclable: fossil-fuel combustion and permanent indirect artificial combustion.
4. OUTPUTS OF CARBON DIOXIDE FROM THE ATMOSPHERE
Outputs of carbon dioxide from the atmosphere are either: (1) biological, (2) geological,
or The main output of carbon dioxide is through biological sequestering. Photosynthesis takes carbon dioxide from the air and incorporates it into the biosphere as organic matter. If the carbon returns to the atmosphere in the short term (mostly through respiration), it is recycled; conversely, if the carbon is not returned to the atmosphere in the short term, it is sequestered. Photosynthesis takes place in terrestrial and aquatic ecosystems, including the oceans. For any ecosystem, rates of biological sequestering are equal to the difference between rates of productivity and rates of respiration. In tropical rain forests, where rates of respiration closely match rates of productivity, there is little sequestering of carbon (Welch, 1992). On the other hand, the photosynthesis of algae and other plants floating in the surface layers of the ocean is more likely to result in sequestering. The respiration of animals in the oceans being less than the productivity, biological sequestering takes place through the settling of dead organic matter to the bottom of the ocean (IPCC, 1996a). Mass balances show that only about half of the carbon dioxide emitted to the atmosphere in industrial times remains in the atmosphere. The unaccounted portion must be going either to increase the biomass of terrestrial and oceanic ecosystems, or to additional sequestering through oceanic-atmospheric interactions. Evidence for the increase of biomass is scant and difficult to document, although the Amazon rain forest, the largest terrestrial ecosystem in the world, appears to be expanding along its perimeter (Prance, 1985). Little is known about a possible biomass growth in the surface layers of the oceans; however, there is reason to suspect that it is there. If indeed the biosphere is growing in the oceans, its effectiveness as a carbon sink would be analogous to that of the cultural eutrophication of lakes and estuaries. Recent efforts to artificially increase oceanic biomass through iron fertilization have been made, although with mixed results to date (IPCC, 1996a). Oceanic/atmospheric interactions are presumed to be responsible for the additional sequestering of carbon (IPCC, 1996a). The ocean is estimated to have taken up about thirty percent of anthropogenic carbon dioxide emissions between 1980 and 1989, thereby slowing the rate of near-atmospheric terrstrial climate change (IPCC, 1996b), while increasing the acidity of the oceans. Anthropogenic reductions in input of carbon dioxide to the atmosphere amount to outputs from the reservoir. Examples are:
5. GEOLOGIC HISTORY OF ATMOSPHERIC CARBON DIOXIDE
Through molecular diffusion, the concentration of carbon dioxide in the atmosphere is nearly constant everywhere. However, its value has fluctuated over geologic time, from as high as 4200 ppmv during the Lower Carboniferous period (345-320 milliion years ago) (Budyko, 1986) to as low as 190 ppmv during the last glacial age (21,000 years ego) (IPCC, 1996a). Long-term variations in carbon dioxide are related to fluctuations in the intensity of volcanic activity (Budyko,1986). The reservoirs of fossil fuels which lie underground developed over a long period of time, starting at the Upper Devonian period (360-345 million years ago) (Williamson, 1967; Magoon and Dow, 1994). As rates of productivity exceeded rates of respiration, effective sequestering of carbon took place, eventualty leading to the formation of fossil fuels.
Over the past 360 million years, the atmospheric carbon dioxide concentration has fluctuated markedly, but the tendency has been generally downwards, from 4200 ppmv to about 200 ppmv. This has allowed the Earth to cool,
setting the stage for Homo sapiens to come of age in the relatively cooler climatic conditions which have prevailed in the past one million years In the last glacial age (21000 years ago), the concentration of atmospheric carbon dioxide reached an all-time low of 190 ppmv. Since then, it has increased slowly, reaching 282 ppmv at the beginning of the industrial revolution, ca. 1800 (IPCC, 1996a). This increase represents an average preindustrial rate of 0.44 ppmv per century, which could be ostensibly attributed to the permanent indirect artificial combustion produced by the agricultural revolution, which began about 11,000 years ago. At the beginning of the 20th century, the carbon dioxide concentration was 299 ppmv, i.e., an increase of 17 ppmv in the 19th century. The present level (1998: 363 ppmv; 2022: 418 ppmv) i.e., an increase of 64 ppmv, which amounts to an average rate of 65 ppmv per century. This last rate is 148 times greater than the average preindustrial rate. Recent rates of increase are even greater; for instance, during 1994, the increase was 1.6 ppmv, i.e., nearly 2.5 times the average rate of the twentieth century. These concentrations are global mean annual values. Thus, they do not reflect seasonal (anual) variations (approximately 1 ppmv), which are partly a function of latitude and altitude (IPCC, 1996a). 6. IMPACTS OF CLIMATE CHANGE Budyko (1986) has estimated that the global mean surface air temperature reached a maximum of 25.5 ℃ during the Lower Carboniferous period. This temperature was 10.6 ℃ higher than at present (Budyko, 1986). Other figures show that a doubling of the current atmospheric carbon dioxide concentration will increase mean global air surface temperature by about 1.5-4.5 ℃, with the best estimate at 2.5 ℃ (Budyko, 1986; IPCC, 1996a).
Major effects of global warming are the melting of the polar icecaps and the resulting rise in global mean sea level. Global mean sea level has risen by 10-25 cm over the past 100 years
In the recent United Nations Climate Change Conference in Kyoto (1997), the United States agreed to reduce its emissions of greenhouse gases by 7 percent (below 1990 levels) by the year 2012. 7. SUMMARY AND CONCLUSIONS
Inputs and outputs of carbon dioxide to the atmosphere are examined using a systemic approach. Geological inputs and outputs are long term, with a time scale of billions of years, nonrecyclable, and beyond the capacity of humans to control. Biological inputs and outputs are short term, with a time scale of years and decades, generally recyclable, and subject to human intervention. The issue of global climate change is essentially one of time scale. Since the year 1900, the human species is effectively acting as an agent of geological change, combusting amounts resembling those of the volcanoes of the past, albeit within a comparatively short time span. Climatic changes which would normally take place over millions of years are instead being accelerated to hundreds of years. In the geological past, carbon dioxide from volcanic eruptions produced sudden global warming, and the biosphere counteracted slowly with global cooling. Cooling has prevailed in the past 360 million years, but warming has prevailed in the past 21000 year, particularly in the past 200 years.
Throughout the eons, the biosphere, with its continuous sequestering of carbon, created the relatively cool climate conditions under which the human species evolved. Through its reliance on nonrecyclable combustion, the human species is now actively engaged in undoing this creation. Thus, we conclude that human societies must reassess their current overreliance on fossil-fuel combustion to support their livelihood. For the latter to become sustainable, it must increasingly tap renewable sources of energy. Given existing global political, economic, and institutional constraints, the implementation of a renewable energy strategy and policy will have to be accomplished gradually. A fifty percent reduction in global fossil fuel combustion (from 1998 levels) by 2025 may be considered reasonable or appropriate. It will be up to the next generation to continue the replacement of fossil fuels with renewable sources of energy. In the meantime, the monitoring of global climate change will continue, the aim being to eventually achieve a measure of stability in the concentration of atmospneric greenhouse gases. Inaction on this issue effectively amounts to continuing an experiment of global proportions. i.e., modeling with the quintessential prototype. Significantly, the other nonrecyclable combustion is permanent indirect artificial combustion, of rates and amounts which are decidedly more difficult to quantify than fossil-fuel combustion. This type of combustion is largely responsible for development as we know it. Thus, for development to become sustainable, it must be redefined in such a way that it minimizes permanent indirect artificial combustion. Such development may prove to be difficult to accomplish. Mitigation and other creative ways of compensating for the destruction or elimination of natural ecosystems may be the only way out of this intensely human predicament. The challenge before humankind is how to achieve economic development without overly relying on fossil-fuel combustion, while minimizing permanent indirect artificial combustion. The engineering of this enlightened type of development is likely to tax the brightest minds of the future. To close, sustainable development maybe unattainable unless global society takes bold and effective steps to effectively reduce its overreliance on nonrecyclable combustion. The issue having a global dimension, the question of environmental ethics arises. Is it right to recognize the global climate change issue now, and act in a concerted effort to resolve it? Or, despite the apparently overwhelming evidence, is it right to continue to ignore the problem for the sake of short-term economic gain? REFERENCES
BUDYKO, M. I. 1977. Climatic changes. Washington, DC: American Geophyscial Union.
BUDYKO, M. I. 1986. The evolution of the biosphere. Dordrecht, Holland: D. Reidel Publishing Company.
CATTERMOLE, P. 1989. Planetary volcanism, a study of volcanic activity in the solar system. New York: Wiley.
HALPERT, M. S., G. D. BELL, V. E. KOUSKY, and C. F.
ROPELEWSKI. 1996. "Climate assessment for 1995". Bulletin American Meteorological Society, 77(5), S1-S44.
HENDERSON-SELLERS, A. 1983. "The origin and evolution of planetary atmospheres". Monographs on Astronomical Subjects, 9, Bristol, England: Adam Hilger Ltd.
IPCC. 1996a. Climate change 1995: The science of climate Change. Intergovernmental Panel on Climate Change, edited by J. T. Houghton et al., Cambridge University Press.
IPCC. 1996b. Climate change 1995: Impacts, adaptations, and mitigation of climate change: Scientific-technical analysis. Intergovernmental Panel on Climate Change, edited by J. T. Houghton et al., Cambridge Univensity Press.
MAGOON, L. B.; and W. G. DOW. 1994. The petroleum system : From source to trap. The American Association of Petroleum Geologists, Memoir 60, Tulsa, Oklahoma.
PRANCE, G. T. 1985. "The changing forests". In: Prance. G T. ; Lovejoy, T. E. Lovejoy (eds.). Oxford, England: Pergamon Press.
UNITED NATIONS. 1994. Combating global warming: Possible rules, regulations, and administrative arrangements for a global market in carbon dioxide emission entitlements. United Nations Conference on Trade and Development, Geneva, Switzerland.
WELCH, E. B. 1992. Ecological effects of wastewater: Applied limnology and pollutant effects. Second edition, Chapman and Hall.
WILLIAMSON, I. A. 1967. Coal mining geology. London, England: Oxford University Press.
WORLD ROAD STATISTICS: 1996. International Road Federation, Geneva, Switzerland.
|
| 221128 |
| Documents in Portable Document Format (PDF) require Adobe Acrobat Reader 5.0 or higher to view; download Adobe Acrobat Reader. |