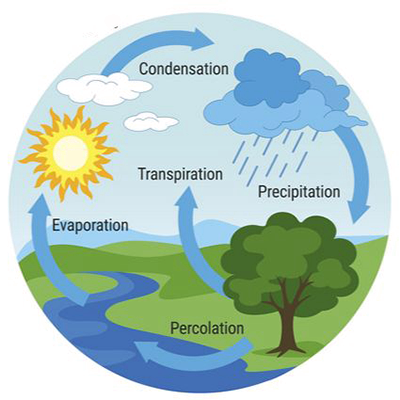
1. INTRODUCTION Water is the quintiessential compound of Nature. It plays an important role in a host of physical, chemical, and biological processes. At the same time, its quantity and quality, including its spatial and temporal distribution, pointedly touch all instances of human endeavor. The study of water continues to engage professionals in a variety of fields, including engineering, physics, chemistry, biology, geology, geography, sociology, and the law, to name some of the most important (Fig. 1). Accordingly, it is an absolute necessity for learned professionals to understand water and its properties, so that its stewardship and management may be acccomplished in a rational way.
The structure of the water molecule appears simple enough, but a complete characterization continues to defy scientists. A systematic study of water, in molecular form and in the aggregate, is much needed to throw additional light onto its capabilities and uses, and to understand why it is the chemical compound chosen by Nature to condition and sustain life. Salient properties of water are the following:
In this article, we endeavor to explore the properties of water with the objective of improving its management.
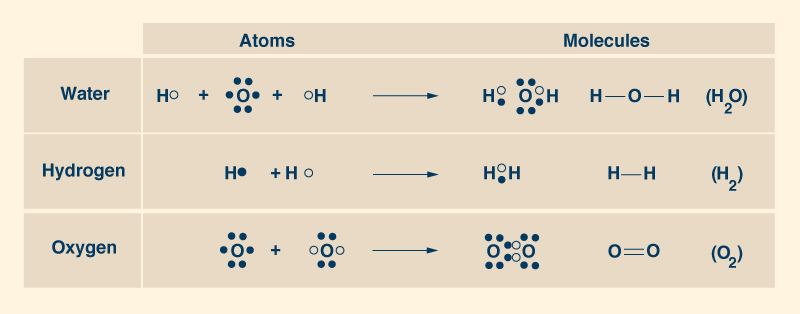
2. THE WATER PIONEERS The French chemist Antoine Lavoisier (1743-1794) discovered that water is composed of two elements: oxygen and hydrogen. Lavoisier recognized the name "oxygen" in 1778 and "hydrogen" in 1783. He gave hydrogen its name, to mean generator of water. In 1804, another French chemist, Joseph Gay-Lussac (1778-1850), together with the German naturalist Alexander von Humboldt (1769-1859), showed that the water molecule consists of two atoms of hydrogen for each atom of oxygen, to form the ubiquitous chemical formula H2O (Fig. 4).
3. THE WATER MOLECULE
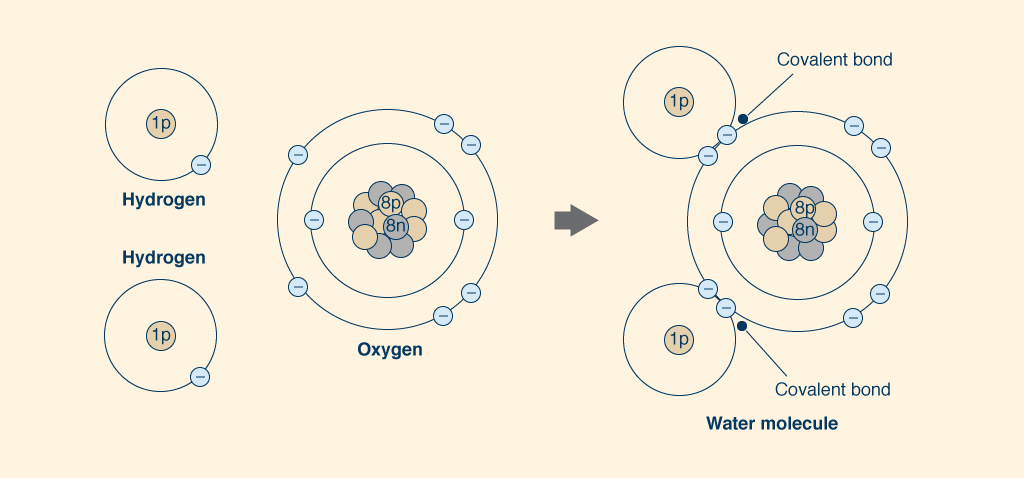
The atoms of water are held together by sharing
their electrons,
the negatively charged subatomic
particles that surround the positively charged nucleus.
The nucleus of each atom of hydrogen (H),
An atom of oxygen (O) has eight (8) protons and eight (8) neutrons in its nucleus, and eight (8) electrons orbiting it, two of which (2) are located in a full inner layer and the other six (6) in an incomplete outer layer, which can take up to eight (8) electrons. Thus, an atom of oxygen can take two more electrons in its outer layer, which participates in the sharing of electrons with another oxygen atom to form the oxygen molecule O2 (Fig. 5). The pairs of shared electrons form paired covalent bonds (represented graphically with two lines like this __), which provide the strongest attraction between atoms, constituting very stable molecules.
To form a water molecule (H2O),
one oxygen atom joins two
hydrogen atoms, proceeding to share their electrons.
Each one of the two
hydrogen atoms shares its lone electron with the oxygen atom in its outer layer, to fill in the
two empty spaces, thus completing the
layer to eight (8) electrons, while forming two covalent bonds (Fig. 6).
In this way, each hydrogen atom is full with two electrons in its single layer,
and each oxygen atom
is full with eight electrons in its outer layer.
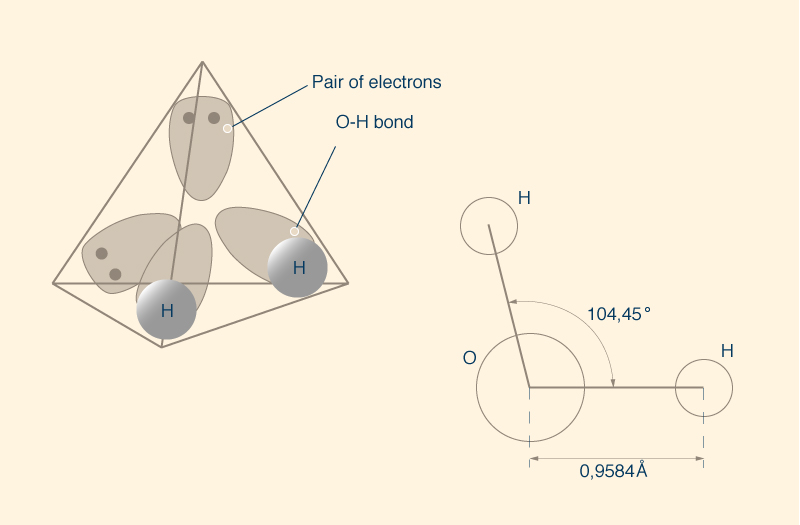
A molecule of water has two pairs of shared electrons, that is, two simple
covalent bonds
If the tetrahedron were a regular one,
the angle between the bonds would be 109.5°;
however, the tetrahedron of the water molecule is not regular.
4. TEMPERATURE REGULATION The range in which water is in liquid state (0 to 100oC at 1 atmosphere of ambient pressure) is ideal for the various life forms that are present on Earth. However, when it is compared with similar compounds, water should boil at a temperature lower than -59°C, not at 100°C. Table 1 contains the values of molar mass and boiling point for several chemical compounds labeled H2X, where X stands for elements located in the same column as oxygen in the Periodic Table of the Elements (Fig. 8). [The molar mass M, in gr/mol, is defined as the mass, in grams, of a given substance divided by its amount, in moles]. Since water has the lowest molar mass among these compounds, the expectation is that it would have the lowest boiling point, not the highest.
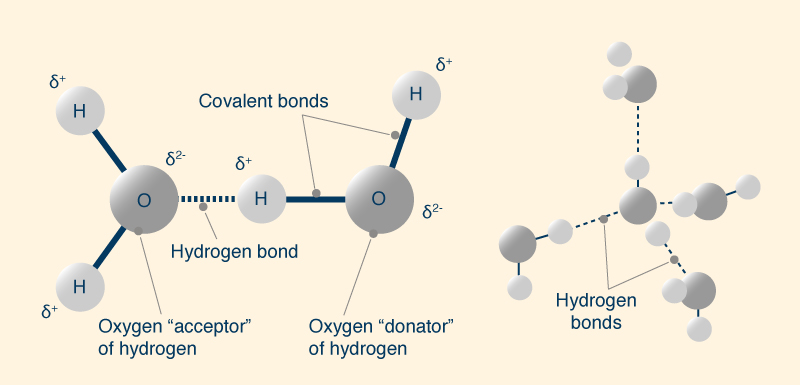
The apparent anomaly can be explained by the
bond between water molecules, or
The hydrogen bond is a weak bond, with a very short lifetime. The broken hydrogen bond, however,
often simply reforms, being broken for very short periods
of time, less than In solid state (ice), all water molecules participate in four hydrogen bonds (two as donor and two as acceptor) and are held in relatively static state. In liquid water, some of the weaker hydrogen bonds break to allow the molecules to move around. This continues to happen with an increase in temperature up to the boiling point. The large amount of energy required to break these bonds must be supplied during the melting and boiling processes. The molecules of H2Te, H2Se and H2S exhibit dipole-dipole intermolecular forces, which are attractive forces between the positive end of one polar molecule and the negative end of another, forming a bond which is weaker than a hydrogen bond.
In summary, a hydrogen bond increases the intermolecular cohesion, which prevents water molecules from
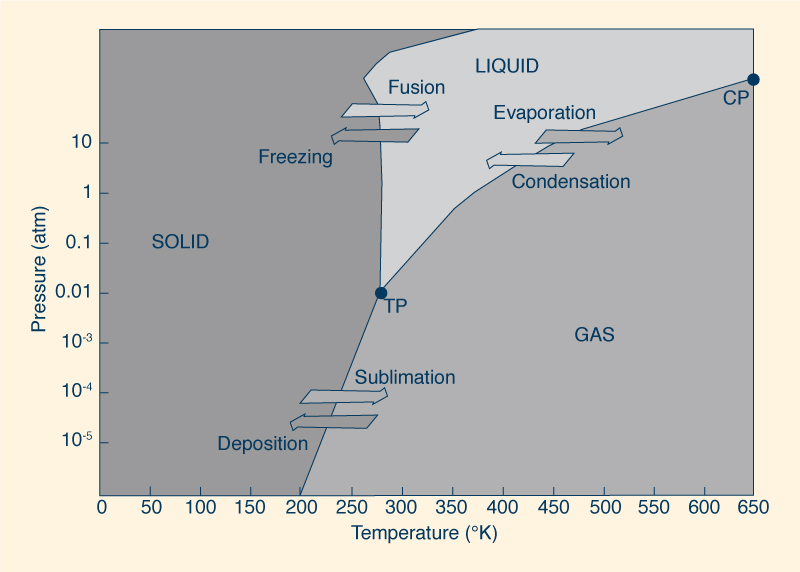
being easily released from the water surface; consequently, the vapor pressure is reduced. The boiling point of water is a function of ambient pressure. For instance, at the peak of Mount Everest, at an elevation of 8 848 m, water boils at 70°C, while in the deep sea it remains liquid above 300°C. To understand this behavior, we must examine the relation between temperature and pressure. The mobility of a water molecule increases with temperature and decreases with pressure. At high altitude, the atmospheric pressure is lower, resulting in more mobility; for this reason, the temperature necessary to reach the boiling point is lower. Figure 10 shows a phase diagram, illustrating the preferred physical states of water at different ranges of temperature and pressure. At TP, or triple point, the three stable phases (solid, liquid, and gaseous) may coexist at equilibrium. On the other hand, at CP, or critical point, the properties of liquid and gaseous phases become indistinguishable from each other. Under sufficiently high temperature and pressure, liquid water is hot enough and gaseous water is under enough pressure for their densities to be equal.
The change of water, from liquid to gaseous phase, occurs with energy absorption (expenditure).
Water has the highest specific heat capacity of all liquids (except ammonia) because a great amount of energy is required to break the hydrogen bonds (Table 2). Since the energy absorbed in this process is not available to increase the water's kinetic energy, it takes a great quantity of heat to raise the temperature of water.
In addition, it takes a great amount of energy to change water from liquid to gaseous state,
because of the energy required to break the hydrogen bonds.
Consequently,
water has the highest heat of vaporization of any liquid and, hence, a very low volatility (Table 2).
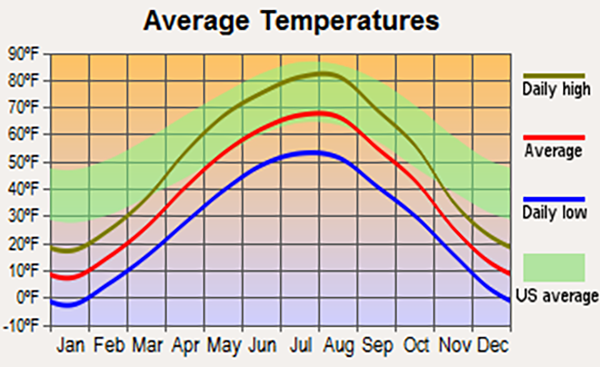
This property of water is important for regional climate regulation, explaining the marked
difference between hyperoceanic and inland continental climates. For
instance, the state of North Dakota, with an inland continental climate,
is subject to more temperature variability between winter and summer than
the entire U.S. average, which is influenced by hyperoceanic climates
5. FLOATABILITY OF ICE
The density of water varies with temperature within
a very narrow range (Table 3).
Typically, liquid
substances shrink with a decrease in temperature, thereby
increasing their densities. However, liquid water presents a
unique behavior, contracting with temperature reduction until the temperature reaches
4°C.
At this point, water reaches the maximum
density of
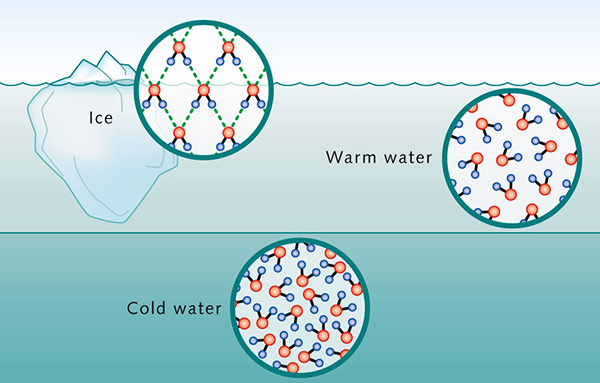
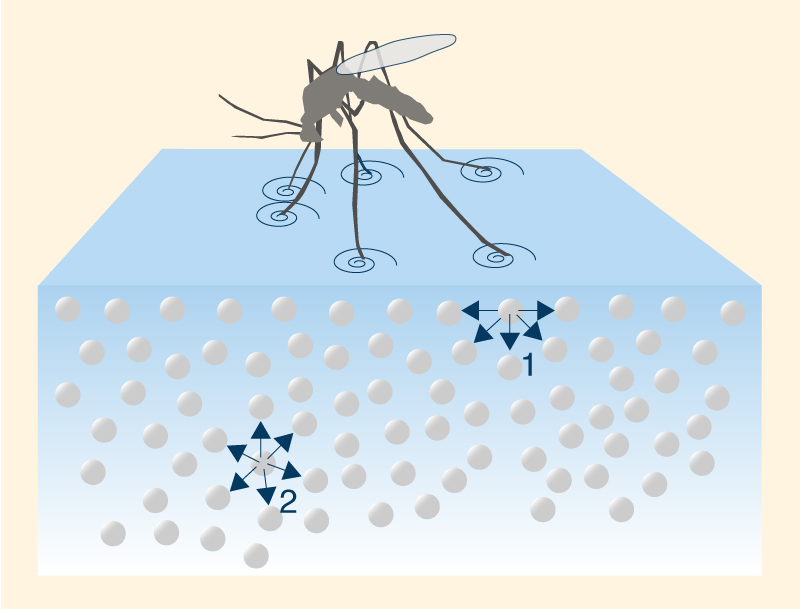
The higher density at 4°C may be explained by analyzing ice structure. The H2O molecules form a hexagonal grid of tetrahedral geometry (Fig. 2). This configuration assures that ice molecules are less compacted than water molecules, occupying a greater volume. Thus, the cooling below 4°C results in the expansion of the space between water molecules, decreasing its density. In Nature, the change in water density with temperature at or near 4°C is responsible for the thermal stratification of the water column. In the absence of mixing, at 4°C the top layer will cool to the freezing point and ice will begin to form on the surface. This ice layer will block the exchange of energy between the cold air above and the warm water below; therefore, the ambient cooling continues, but with no drop in temperature of the water column below. The formation of an ice layer on the surface of the water body discourages the freezing of the water column; therefore, it makes possible the survival of aquatic plants and animals in lakes and seas. 6. SURFACE TENSION AND CAPILLARITY Due to high cohesive forces, water molecules at the surface are more attracted to the molecules within the liquid than they are to molecules (of air) outside of it [1], producing surface tension at the gaseous-liquid interface. In contrast, molecules of water inside the liquid [2] are equally attracted in all directions. This property makes it more difficult to move an object through the surface than to move it when it is completely submerged. That is why insects, which are heavier than water, can float and slide on a water surface (Fig. 12).
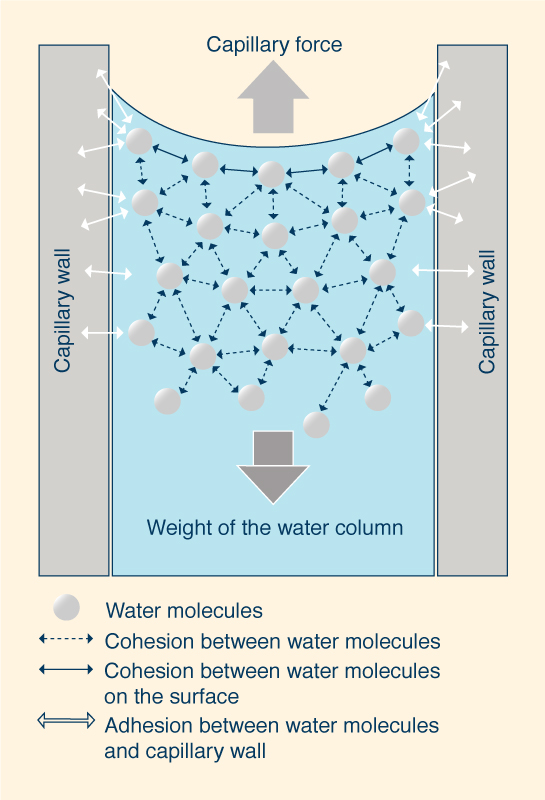
The movement of water up or down capillary tubes is due to surface tension.
Capillary action occurs when the adhesion of water molecules to the
walls is stronger than the cohesive forces between the liquid molecules.
The meniscus is the curved liquid-gaseous surface interface;
it is concave when the liquid molecules are strongly attracted (due to adhesion) to
the walls of the container, as in the case of water
Capillary action makes it possible for plants to thrive. Capillarity allows subsurface water to move up into the root zone, but only up to a small distance, after which it cannot overcome gravity. Due to strong cohesion forces, the water molecules that evaporate on the leaf surface attract others in the neighborhood, helping the water to get up the plant, eventually reaching all branches. Within the soil profile, the capillary height is 2 to 5 cm in coarse sand, increasing as the soil particle diameter decreases, reaching more than 3 to 4 m in some cases. 7. POLARITY AND SOLVENT PROPERTY
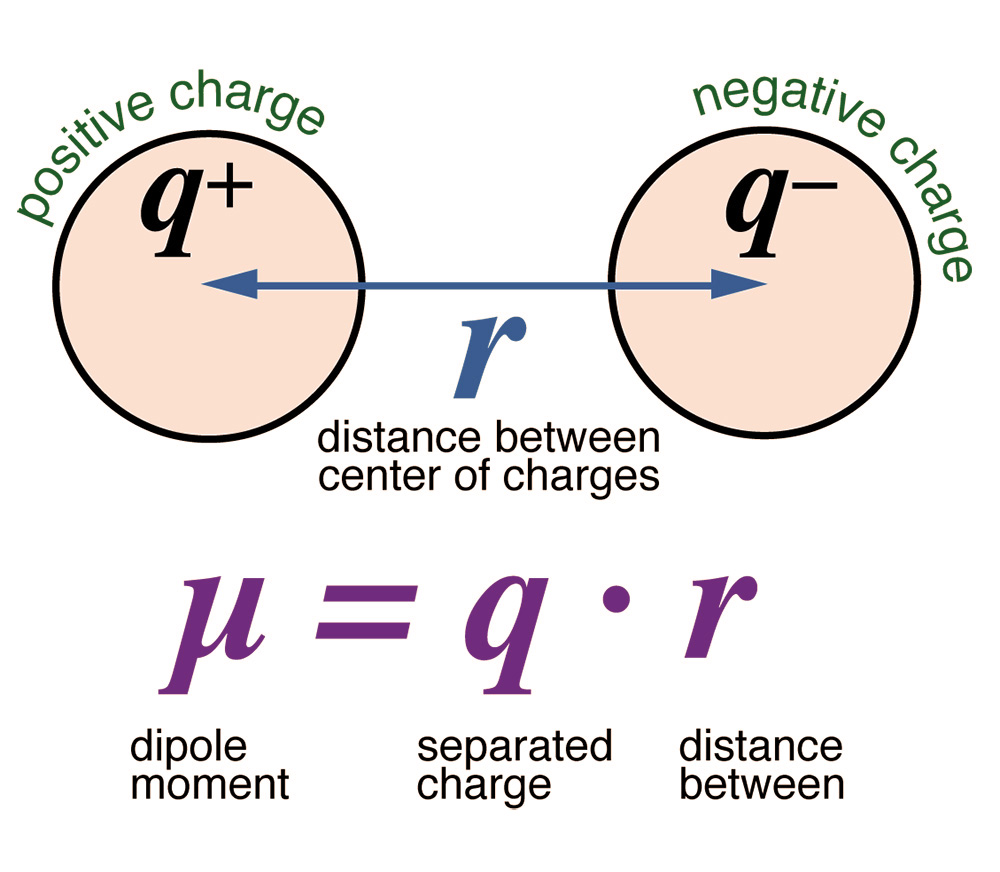
The water molecule has the property of polarity,
featuring a relatively large dipole moment (Fig. 14).
The polarity of water is important for many of its properties, including the ability to dissolve solutes, melting and boiling points, specific heat, surface tension, and general reactivity. Water can dissolve almost anything and, therefore, it is regarded as the universal solvent.
Water's large dipole moment is associated with
its relatively high dieletric constant. Coulomb's Law,
named after the French physicist Charles de Coulomb (1736-1806)
In a vacuum, k = 1; for water, k = 78.5 (Table 4). This large value of k, derived from the water molecule's polarity, makes it possible for the substances formed by the association of two charged components (most notably, sodium chloride) to disassociate readily in water, and that all polar substances see their attraction and repulsion forces diminished. Moreover, the presence of dissolved ions in water results in a marked increase in its electrical conductivity; otherwide, water would behave essentially as a nonconductive medium because of the patent lack of native ions in its pure state.
In salt water, electricity is conducted by ions. Sodium ions absorb electrons from the negative terminal, passing them to chlorine ions and then to the positive terminal, forming a bridge which conducts electrical current. Distilled water has a very low electrical conductivity of 0.000055 dS/m (deciSiemens per meter), potable water is around 0.1 to 0.5 dS/m, and seawater about 44 dS/m. Therefore, the greater the salinity of the water, that is, the greater the concentration of ions in the solution, the greater its electrical conductivity. The relation between salinity and electrical conductivity may be calculated using Online Salinity. Life, which is based on carbon chemistry, requires a liquid medium to develop effectively. Water in its liquid state is the best medium for that chemistry. Water is required for the dissolution of chemical compounds that sustain life and for the transport of nutrients and wastes. These compounds include salts, sugars, acids, bases, and gases such as oxygen and carbon dioxide. In addition, water associates itself to the polar regions of organic compounds such as lipids, proteins, and nucleic acids. Often these compounds form hydrogen bonds with water, which are weak attractions between a proton in one molecule and an electronegative atom in the other. 8. PROTON CONCENTRATION The energy driving biological processes is generally due to gradients of proton concentration in an aqueous medium, on both sides of a membrane. A proton of hydrogen (H+) is an atom of hydrogen which is missing its electron. The water molecule has a weak capacity to spontaneously separate into two distinct ionic components: (1) the hydronium ion, or positively charged cation (H3O+), which is a water molecule with an attached hydrogen proton; and (2) the hydroxide ion, or negatively charged anion (OH-), which is a water molecule with a hydrogen proton missing, in a reversible formula:
In pure water, the concentrations of hydronium and hydroxide ions are very small and identical,
each being The pH of water decreases sharply in a solution containing large quantities of a strong acid such as hydrochloric acid (HCl). The molecules of HCl readily donate their protons to increase the concentration of hydronium ions, i.e., decrease the pH of the solution.
Likewise, the pH of water increases sharply in a solution containing large quantities of a strong base such as sodium hydroxide (NaOH). The molecules of NaOH readily accept protons (or donate hydroxides) to decrease the concentration of hydronium ions, i.e., increase the pH of the solution.
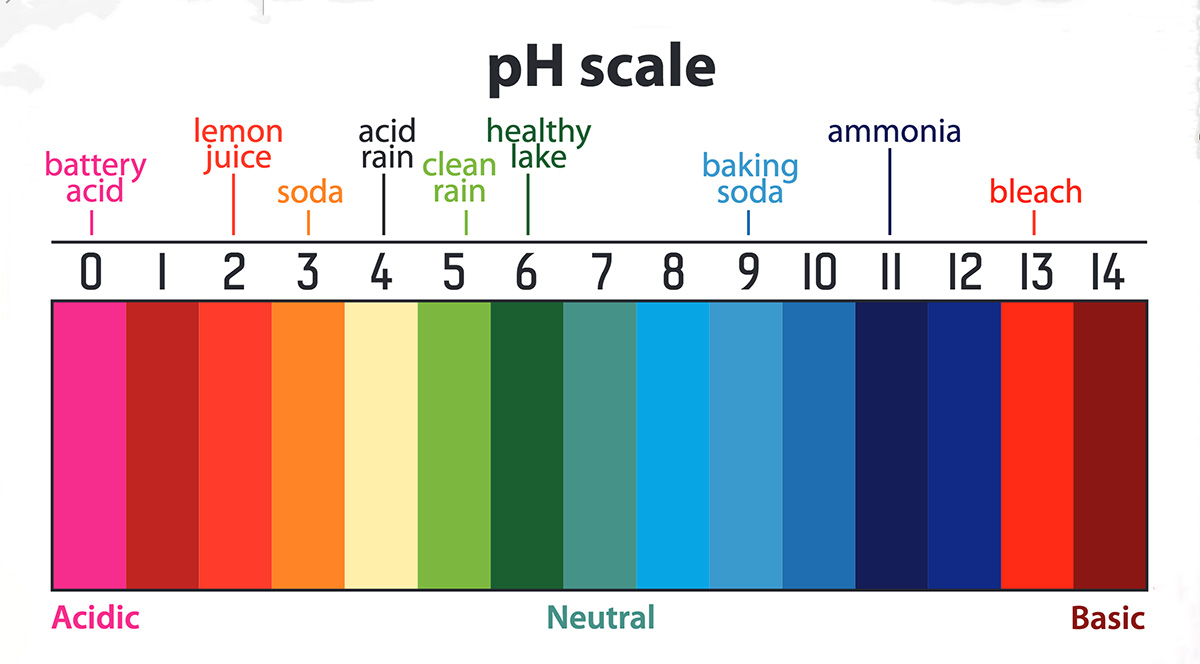
All organisms have a range of pH in which their body fluids remain healthy. Values of pH too high or too low may cause damage to cells. Normal values generally tend to be close to neutral, i.e., pH = 7. Figure 16 shows some typical aqueous solutions in a pH scale.
9. ELECTRON CONCENTRATION
The concentration of electrons in an aqueous medium is useful in characterizing the various
stages
of the oxidation/reduction process. For this purpose, the concept of redox potential, or
oxidation-reduction potential (ORP), is used.
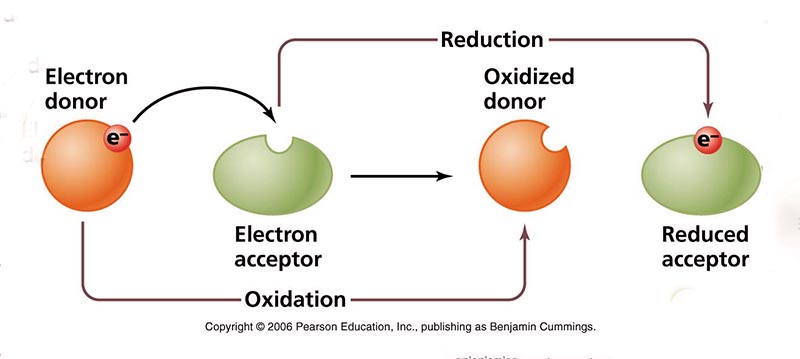
Redox potential is a measure of the affinity of a substance
to lose or gain
electrons and, thereby, to be oxidized or reduced, respectively (Fig. 17).
The standard is hydrogen, with zero redox potential, or
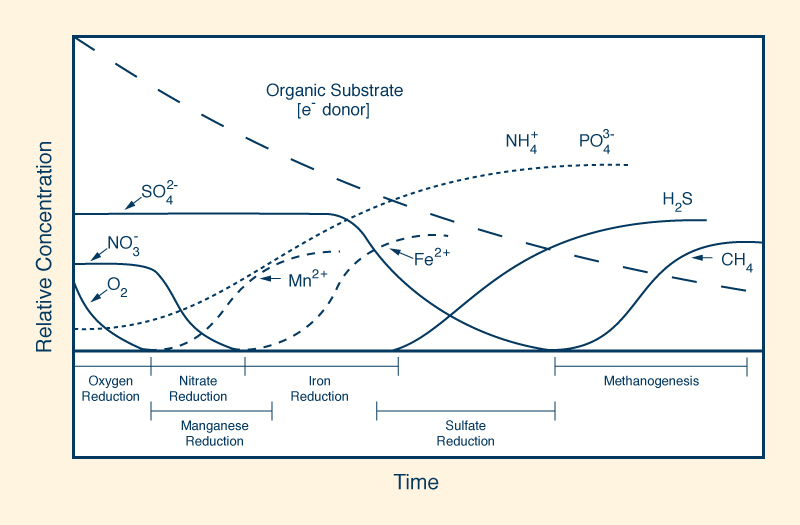
A positive value of redox potential indicates that a substance is an oxidizing agent; the higher the reading, the more oxidizing it is. As such, a substance with a reading of +400 mV is four (4) times more oxidizing than a substance with a reading of +100 mV. Conversely, a negative reading indicates that a substance is a reducing agent; the lower the reading, the more reducing it is. Thus, a substance with a reading of -400 mV is four (4) times more reducing than a substance with a reading of -100 mV. Most types of water, including tap water and bottled water, are oxidizing agents as their value of redox potential is positive. Alkaline ionized water is a reducing agent, as it has a negative value of redox potential and it is able to donate extra electrons to neutralize the harmful effects of free radicals on the organism. However, most other types of water are oxidizing agents as their redox potential is positive. In wetland ecosystems, the redox potential conditions the time sequence of various types of oxidation-reduction reactions in newly flooded organic substrate, ranging from oxygen reduction at highly positive redox potential (E ≈ 800 mv), to methanogenesis at very low negative values (E ≈ - 400 mV). Figure 18 shows the decrease, with time, of the relative concentration of electrons in seasonally flooded soils.
10. CONCLUDING REMARKS
The physical, chemical, and biological properties of water are reviewed
with the aim to understand the diverse role of the water molecule in conditioning and supporting life.
The singular properties of water make it well suited for
the development of carbon chemistry, helping sustain
life in all of its myriad forms.
Therefore, the properties of water span physics, chemistry, and biology, as if Nature had
intended for References
Aguilera Mochón, J. A. 2017. El Agua en el Cosmos: La Matriz de la Vida.
RBA Coleccionables, Navarra, Spain.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 220829 |