|
1. MEASUREMENT OF SALINITY
-
The chemical and physical properties of a soil affected by salts reflects the amount and type of salt present.
-
The use of salinity meters in the field is becoming more and more common.
-
Laboratory analysis of aqueous soil extracts remains the most common technique for assessing salinity.
-
The electrical current carrying capacity of a solution is proportional to the ion concentration in the aqueous solution.
-
Electrical conductivity is measured in ohm-1 or mho.
Conductivity is the reciprocal of resistance.
-
In SI units, one mho is designated as Siemens (S).
-
Electrical conductivity is measured in S/cm or, alternatively, in milliS/cm
(1 milliS/cm = 0.001 S/cm).
-
One milliS/cm is equal to one deciS/m, or, for short, dS/m.
-
Calculation of total dissolved solids TDS (mg/L) based on electrical conductivity
EC (dS/m)
2. SALINITY AND SODICITY
-
A soil is considered saline if the electrical conductivity of a saturation extract exceeds the value of 4 dS/m at 25°C and the percentage of the cation exchange capacity of the soil occupied by sodium is less than 15.
- The value of 4 dS/m corresponds
to approximately 40 meq/L of salt.
- Sodicity is estimated from
the exchangeable sodium percentage (ESP) and the sodium absorption ratio (SAR).
- ESP is the percentage
of the cation exchange complex occupied by sodium ions.
- Soils with ESP greater than 6 are sodic.
- Soils with ESP greater than 15 are strongly sodic.
- The SAR is the ratio of the Na concentration to the square root of the average Ca and Mg concentrations.
- SAR values greater than 15 indicate sodicity.
3. INSTRUMENTS TO MEASURE SALINITY
-
The four-electrode salinity probe is a probe of small diameter with four electrodes spaced a few centimeters apart along its length of about 150 cm (Fig. 1).
Fig. 1 La sonda de salinidad de cuatro
electrodos.
-
Two of the electrodes create an electric field.
The other two measure the electrical resistance of the soil.
-
To use it, the probe is inserted into the soil to the desired depth. The volume sampled is about 90 cm3.
4. WEATHERING
- Soils in arid and semiarid regions are relatively unweathered.
- Unweathered minerals provide plant nutrients, but are also a source of soil salinity.
- Increases in salt content of 200 to 300 mg/L are common when arid-land soil solutions remain in contact with relatively unweathered soil minerals for substantial periods of time.
- The amount of salt dissolved under such conditions depends on the quantity of carbon dioxide.
- The partial pressure of carbon dioxide can reach 100% or more when oxygen is consumed and carbon dioxide released during soil respiration.
- Dissolution of primary minerals is most important when the irrigation water's salt content is low, less than 100 to 200 mg/L, or when the leaching fraction is at least 0.25.
- Irrigation with water from California's Feather river, which has a salt content of 60 mg/L, results in more salt in the drain water due to weathering than due to the salt content of the irrigation water.
- For salt-affected soils, mineral weathering is seldom a significant part of salt balance computations.
- When irrigation waters have a moderate amount of salt, such as the 800 mg/L that occurs in the Colorado river lower reaches, and leaching fractions are below 0.25, salts precipitated in the soil profile exceed the amount weathered.
- At low leaching fractions, LF = 0.1, 20% or more of the salt in irrigation water precipitates and is not contained in the drainage water.
- Salt removal by crops (crops taking up salt) is too small to affect the salt balance.
- The deeper the soil, the greater the capacity to store salt with minimal yield reduction.
5. LEACHING REQUIREMENTS
-
The amount of leaching needed to maintain a viable irrigated agriculture depends on the salt content of the irrigation water, soil, and groundwater; the salt tolerance of the crop; the climate, and soil and water management.
-
The only economical way to control soil salinity is leaching.
-
Definitions of leaching fraction:
-
Ratio of depth of drainage to depth of applied water (irrigation plus rainfall).
-
Ratio of salt content of applied water to salt content of drain water.
-
Ratio of electrical conductivity of applied water to electrical conductivity of drain water.
6. SALT TOLERANCE
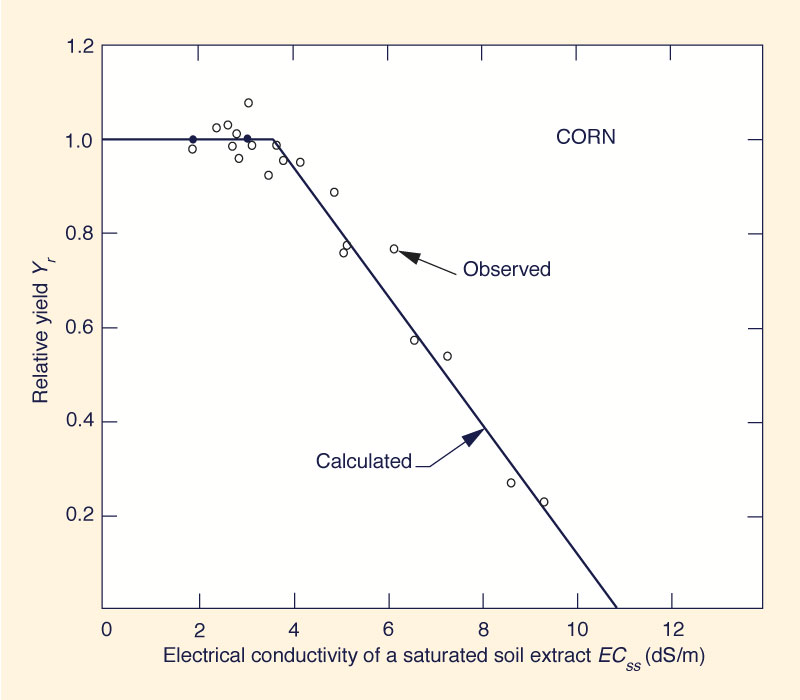
- The salt tolerance of a crop can be described by plotting the relative yield as a continuous function of soil salinity.
- For each crop, there is a maximum soil salinity, the threshold dS/m, that does not reduce yield (for example, a value of ECss = 3.6 for corn, shown in Fig. 3).
Fig. 2 Variation of relative yield
of corn with soil salinity.
-
The threshold dS/m varies from 1 to 8 dS/m.
-
Sodium is not considered an essential element for most plants, but it beneficially affects the growth of some plants at concentrations below threshold dS/m.
- Injury by sodium to avocado in avocado and citrus is widespread; it causes leaf burn (Fig. 3).
- Chloride is an essential micronutrient for plants, but, unlike most macronutrients, it is relatively nontoxic.
- Chloride contributes to osmotic stress.
Fig. 3 Leaf burned due to excess of sodium.
7. LEACHING FOR SALINITY CONTROL
- The volume of water needed for a given degree of leaching may be greater for surface irrigation that for sprinkler irrigation.
- The minimum depth of water that can be applied uniformly by surface methods is several times greater that the minimum for sprinkler irrigation.
- Salts should be carefully monitored by direct soil measurements or, frequent, careful observations of crop conditions.
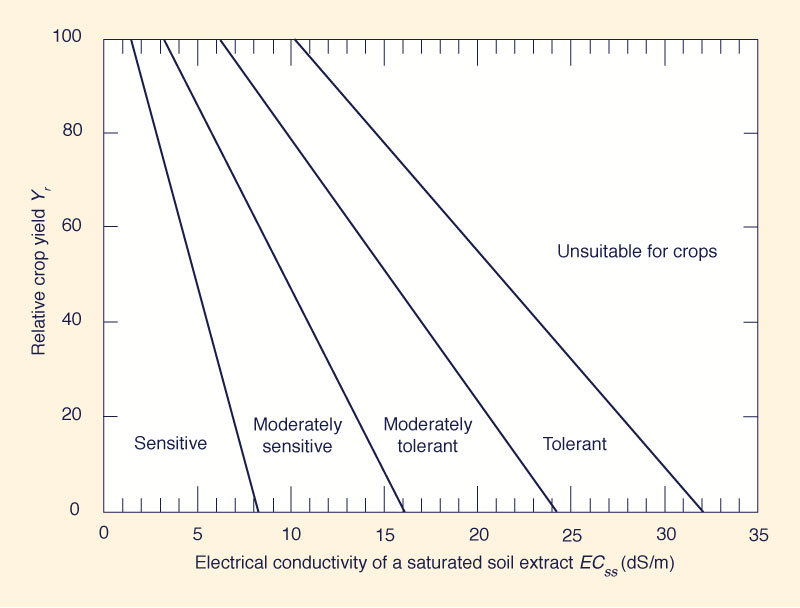
- Tolerance to salinity depends on each plant (Fig. 4).
Fig. 4 Plant tolerance to salinity.
- For example, given a value of ECss = 10 dS/m:
- A sensitive crop would have Yr = 0%;
- A moderately sensitive crop would have Yr = 47%;
- A moderately tolerant crop would have Yr = 78%; and
- A tolerant crop would have Yr = 100%.
|