IS THE IRRIGATION OF ARID LANDS A DOUBLE-EDGED SWORD?
Professor Emeritus of Civil and Environmental Engineering
San Diego State University, San Diego,
California
1. INTRODUCTION
Nature has time; humans do not.
In the recognition of this truism lies the story we are about to tell.
Two important issues may not have been fully recognized at the time: (1) arid lands naturally contain more salts than humid lands, and
(2) if the newly available salts are allowed to sit around, eventually they are bound to reach the
groundwater reservoirs, contaminating them with additional salinity for the foreseeable future.
2. NATURE'S WAY
The oceans contain large quantities of salt ions, about 3.5% by weight, 35‰,
or 35,000 ppm, mostly ions of sodium (Na+) and chloride (Cl-).
This salt originated in the rock mantle,
mobilized by leaching from the soil profile to the adjacent surface waters,
and accumulated in the oceans throughout eons.
The quantity and quality of nutrients, and their related ions (cations and anions),
are sourced in the originating parent rock.
Four cations (from the group of Alkali and alkaline earth metals)
readily stand out due to their wide availability and
relative importance in life processes:
This is where the similarities end. Vegetative ecosystems are known to be highly
selective in their use of these four common salt cations;
they readily uptake magnesium and potassium, while largely wasting sodium and calcium.
This selectivity manifests itself after leaching to the river waters,
where the percentages of sodium and calcium together rise to about 24% of all salt ions,
while magnesium and potassium jointly constitute only about 7%
(Table 3).
Therefore, sodium and calcium are delivered to the oceans in much greater
quantities than magnesium and potassium.
Once in ocean waters, calcium (Ca+) is taken out of solution by biological organisms
for the building of shells and skeletons (Fig. 1)
or by chemical precipitation, which leaves sodium to constitute
the lion's share (84%) of the four common salt cations in ocean waters
(Table 4c).
Together with its ubiquitous partner
the chloride (Cl-) anion, sodium and chloride
constitute about 86% of all the salt ions present in ocean water
(Table 3).
Therefore, throughout the passage of eons,
the selectivity of the biosphere, with regard to the four common salt cations, has largely
determined the present composition of the oceans' chemistry.
In summary, sodium and calcium are wasted by the terrestrial biosphere, eventually
flowing into the oceans, where calcium is largely
taken up by ocean biota. This leaves sodium, and its partner, chloride, as the only true wastes
of Nature, left to accumulate in the world's oceans, thanks to gravitational forces, through geologic time.
3. HYDROLOGY AND SOIL FERTILITY
Water has a
very strong capacity to pick up solids.
This is due to its small but appreciable
dipole moment,
All soils originate in the parent rock. They are produced by weathering and subsequent erosion, transported and deposited in the valleys, where the flowing surface water loses speed and ultimately deposits its load of sediments (boulders, gravel, sand, silt, and clay). The soil particles are loaded with nutrients, both internal, inside the soil particle's matrix, and external, on the surface. At the time of soil formation, the quantity and quality of nutrients depended on the geological origin of the parent rock, whether igneous, sedimentary, or metamorphic. To an extent, it is also related to the luck of the draw: some soils contain specific quantities of certain nutrients; for instance, an example is the prominent presence of selenium in California's Central Valley, as well as in other regions of the Western United States (Water Education Foundation, consulted on September 9, 2023).
The water molecule's large dipole moment is responsible for setting
in motion the continuous interaction of hydrologic science
with soil science:
The greater the amount of mean annual precipitation, Conversely, the smaller the amount of mean annual precipitation, the lesser the possibility of leaching of the underlying soils. Therefore, semiarid to superarid climates find their soils filled with nutrients, many primeval, i.e., hardly, if ever used. The drier the climate, the greater the amount of nutrients likely to be stored in the soil profile. In this case, the dictum changes to: The lesser the amount of mean annual precipitation, the greater the amount of nutrients.
Figure 3 shows a paired composition of two quite
different situations. Figure 3 (a) is a view of
the Sahara desert, with mean annual precipitation of 76 mm, definitely a superarid climate.
Figure 3 (b) is a view of the Amazon rainforest, with mean annual precipitation of 2,600 mm,
reflecting a decidedly humid climate.
Experience has shown that while the soils of the Sahara desert are
loaded with fresh nooutrients,
The palm tree of the Sahara desert is able to tap the scant amount of soil moisture above the groundwater table and proceed with its physiological growth needs [Fig. 3 (a)]. Despite its apparent lushness, the Amazon rainforest has no other choice but to store the ecosystem's available nutrients within its abundant canopy and litter, to recycle them as appropriate [Fig. 3 (b)].
Within reasonable limits, just about any agricultural crop
may be able to prosper in the Sahara desert, if only there was
enough water to allow limited evapotranspiration to take place.
4. GEOMORPHOLOGY AND DRAINAGE
Enter the important science of
geomorphology. Empowered with the force of gravity, Nature has
created two types of drainages on the surface
of the Earth: (1) peripheral, and
Exorheic, or peripheral
drainage basins are located in the periphery of continents.
They carry the surface waters from headwaters to ocean,
delivering their load of salts and closing the hydrologic cycle.
Endorheic, or non-peripheral drainage basins
do no such thing;
instead, they drive themselves to a low spot in the landscape,
where runoff collects and is subject to
evaporation. Eventually, the moisture returns to the atmosphere,
shortcutting the hydrologic cycle and leaving the load of salts somewhere
within the basin confines, to sit there for the foreseeable future.
The consequences of this situation may not be altogether apparent. Peripheral basins are normally in salt balance;
i.e., experiencing no salt accumulation, because the new salts generated over time
are being continuously carried out to the ocean. On the other hand,
non-peripheral basins defy salt balance, with
the amount of salt deposited within their confines
forever increasing as time continues to produce more salt.
There is no salt flushing in a non-peripheral basin.
Peripheral basins thrive because the small amount of salt in the environment
is technically background noise,
an amount that is not increasing with time and that the ecosystem
may have already gotten used to.
The existence of an endorheic basin amounts to the
proverbial luck of the draw.
Given the harsh reality of tectonism, the larger the continent, the more the likehood
that its centrally located, or middle regions,
would be endorheic. Just about every continent
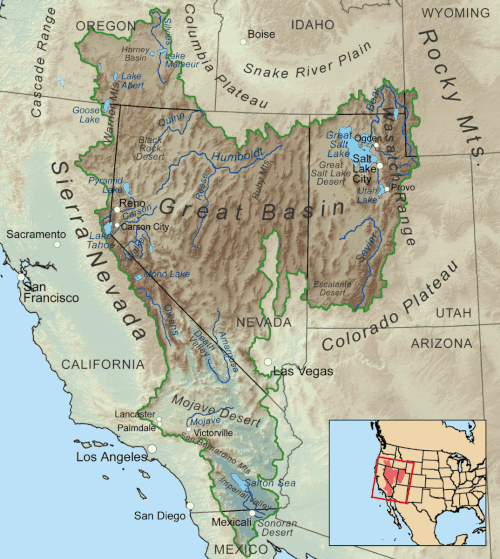
has its share of endorheic basins. For example, in the United States, the Great Basin
comprises most of the state of
Nevada, half of Utah, substantial parts of
California, and smaller portions
of Arizona, Oregon, Idaho, Wyoming, and the states of
Baja California and Sonora, in neighboring Mexico.
The basin covers about 6.4% of the
conterminous 48 states, not accounting for the small area in northern Mexico.
It should not escape anyone's attention that the salt currently in the oceans most likely did not originate in endorheic basins. Endorheic basins collect salt, with some notable exceptions. It would take a cataclysm of near biblical proportions for any of that salt to be delivered to the oceans. A case in point: About 15,000 years ago, ancient Lake Bonneville, in Utah, breached its northern edge and flowed into the Upper Snake river, in the Columbia river basin, eventually losing about 84% of its surface area while lowering the water surface elevation by about 400 m! [Click -here- to watch a 2012 video describing the event].
5. LAKE SALINITY
The salinity of the oceans hovers around 35‰,
varying from 32‰ to 37‰, depending on
local precipitation, evaporation, and runoff
from nearby major sources of fresh water (the mouths of large rivers). Other terrestrial bodies of water, such as lakes,
display a much broader range of variation in salinity, reflecting a complex array of factors, including
geologic age, local and regional ecology and geomorphology and, more recently,
antropogenic influence.
One observation may be drawn from the data. Lakes in arid regions tend to have high salinity,
while lakes in humid regions display an opposite trend. Two cases
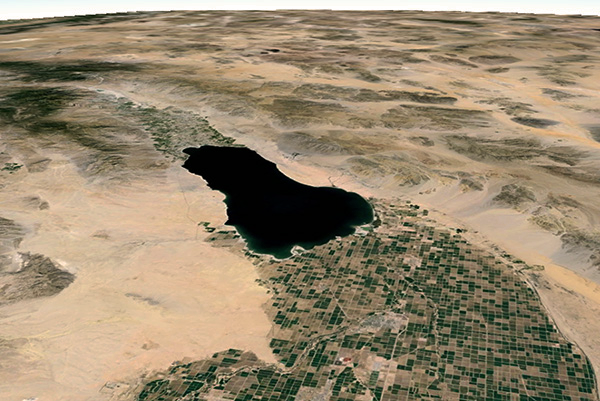
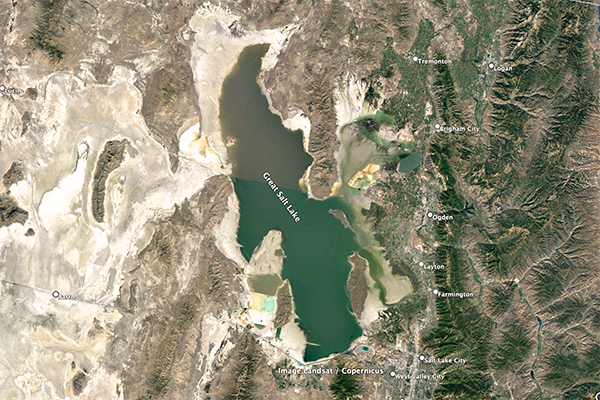
are described here: (1) Great Salt Lake,
Great Salt Lake is an endorheic
hypersaline lake, its salinity
varying between 50 and 270‰, depending on location and lake level
[For reference, at 25°C, 357‰ is the salinity concentration at
which sodium chloride precipitation begins].
In contrast to the Great Salt Lake, the Great Lakes of North America are partially exorheic.
We conclude that the salinity of a lake such as the Great Salt Lake (50‰ at its low end)
is about
Fig. 5 (a) The Great Salt Lake, Utah.
Fig. 5 (b) Lake Superior, Ontario and Michigan.
In closing, lake salinity depends on: (1) the extent of its exorheism,
and (2) the climate, ecology, and geomorphology of the surrounding environment.
6. SOILS AND DRAINAGE
The extra salt present in a given soil profile,
which is mobilized by subsurface runoff into local and regional drainage, may originate in one of the following
sources: (1) old natural salts; (2) new natural salts;
Old natural salts are those typically of marine or lake origin,
actually present in the soil, typically as salt layers, on account of the geologic history of the region
[Fig. 6 (a)].
New natural salts are those that were originally inside
the soil matrix and were mobilized by irrigation, the addition of moisture, biological weathering,
and the partition of sand-sized particles.
Old artificial salts are those that are brought in with the irrigation water, typically
from a distant place, labeled "artificial" due to their
anthropogenic origin [Fig. 6 (b)].
New artificial salts are those that came with on-farm fertilization, due to the
perceived need to increase the productivity of the irrigation enterprise.
An enlightened irrigation project makes every effort to properly account for
a salt budget.
At this point, it bears to reiterate that the irrigation of arid lands
effectively creates new salts by partitioning the relatively young
soils to extract the useful
salts (magnesium and potassium), while disposing of the waste salts
(sodium and calcium),
the latter to be removed by appropriate drainage [Fig. 6 (b)] (Rhoades and others, 1968).
There is no way out of this predicament.
The more arid the irrigation site,
the more waste salts will be mobilized, creating the need to be eventually
removed from the premises.
7. THE DESIGN OF NATURE
The rivers of the Earth are responsible for carrying the surplus salts to the ocean.
Water's dipole moment ensures that the salts are picked up by both subsurface and surface
runoff.
Without runoff, the delivery of waste salts to the oceans would not have been
possible.
But runoff is always there, particularly for peripheral basins in salt balance [A basin in salt balance
is one with no significant salt accumulation over time].
About 40% of the precipitation (rainfall)
falling onto the Earth's land surface actually makes it to the oceans,
28% as surface runoff and 12%
as subsurface runoff
Runoff has a salinity concentration, i.e.,
the dissolved excess salts that were picked up somewhere upstream.
The partnership of water and salt having been established, we now turn our attention to the water, i.e.,
to the surface runoff. On a global average basis, we have noted that
about 40% of terrestrial precipitation is actually
converted to surface runoff and delivered to the oceans. This figure,
referred to as runoff coefficient, is a temporal and spatial average.
Across the world,
runoff coefficients vary widely, from as low as 2% in some
extremely arid regions to 93% in certain very unusual
geological settings (L'vovich, 1979). Consider, for example,
the following statement:
In Nature, runoff coefficients have a tendency to increase with the amount of environmental moisture.
Terrestrial and lake/reservoir evaporation, together with plant transpiration,
constitute total evapotranspiration. The latter has the net effect of concentrating the salts,
since these are left behind in the water that remains
A global value of runoff coefficient of 40% means that the remaining 60% was actually
consumed and returned to the atmosphere by:
(1) land evaporation, (2) water-body evaporation,
and
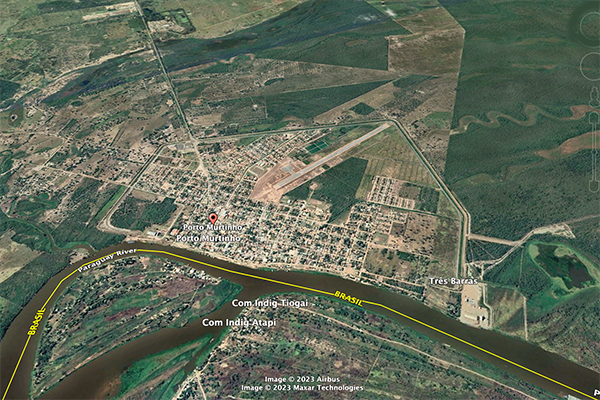
A case in point: The runoff coefficient
of the Upper Paraguay river, measured at Porto Murtinho, in Mato Grosso do Sul, Brazil,
near its mouth at the confluence with the Apa river,
should be above
Fig. 7 (a) Porto Murtinho, in Mato Grosso do Sul,
Brazil, surrounded by a polder for flood control purposes,
Fig. 7 (b) Fecho dos Morros, in Mato Grosso do Sul, Brazil, literally
Closing of the Hills, a tectonic uplift feature
8. THE DESIGN OF HUMANS
Enter humans. For the past more than 100 years,
contemporary human societies have been
engaged in altering the water balance of a
basin by converting runoff into evapotranspiration by way of irrigation.
There is a lofty aim to this endeavor: To help sustain human populations by the cultivation and harvest
of additional amounts of food and fiber.
A common belief
is that the water resources of a region are precious,
and should not be wasted by letting them flow into the ocean.
Irrigation converts water that would otherwise flow into the ocean
into additional evapotranspiration. This has the net effect of
reducing the amount of runoff and
increasing the concentration of salts in the drainage waters.
Over the years, irrigation has seemed to work well, and it has led to some successes, many of which
have been documented in the literature.
In general, however, irrigation is not without its pitfalls
Not all irrigation projects produce a comparable amount of waste salt. The quantity of waste salts
tends to be much larger in arid regions than in humid regions. A well managed irrigation project
develops a salt budget to account for all the sources of salts, calculates the amount of salts
that are to be wasted, and formulates a strategic plan for salt removal and disposal.
After irrigation projects were developed in earnest,
the realization of the need for salt disposal encouraged the development
of the field of irrigation drainage engineering [Fig. 8 (a)].
At great expense, the waste salts may be removed from the premises, but only at the cost of
increasing the
salinity of the flow (channel, stream or river) that is charged with removing the waste salts and
conveying them
to their final destination. There does not seem to be a way out of this predicament!
The disposal problem may be somewhat
manageable when the irrigation project site is reasonably close to the nearest ocean;
say, along the coast, or within a short distance from it.
Otherwise, a difficult problem of waste salt disposal arises, one that initially may be characterized as
a technical problem, but that eventually becomes a political problem.
Two examples in California, those of the San Joaquin valley
(irrigated since the 1800s) and the
Imperial valley (irrigated since the early 1900s) help
describe the experience
with irrigation drainage waters
(Ponce, 2005;
2007).
Rather than sending the waste salts all the way to the ocean, a solution may be to store them in evaporation
ponds, where they may remain forever hidden from general view [Fig. 8 (b)]. However, this solution is
not good in the long run, because the stored waste salts are bound to eventually seep into the
local/regional groundwaters and contaminate them with additional new salinity
Fig. 8 (a) Irrigation and drainage canals,
Wellton-Mohawk Irrigation District, Wellton, Arizona.
Fig. 8 (b) South evaporation pond, Tulare Lake Basin,
Given enough time, likely to be measured in centuries, irrigation could end up
replacing healthy peripheral basins with pockets of salt-infested endorheic basins,
purportedly to hold for the foreseeable future the waste salts produced by irrigation.
The environmental impact of such change, likely to encompass many generations,
will prove to be very difficult to manage.
Case study: The Salton Sea, in California.
Waste salts have been collected in this below-sea-level (-96 m) large repository of agricultural drainage
for a little
more than a century. An effective solution for the many
problems that the Sea faces, and one that will
satisfy all stakeholders, will very likely be prohibitively expensive,
given that too much time
has gone by and large amounts of salt have accumulated.
9. SUMMARY
The findings of this article may be summarized in the following points:
Vegetative ecosystems are known to be highly selective in their use of the four common
salt cations; they will readily uptake magnesium and potassium, while
largely wasting sodium and calcium.
Once in ocean waters, calcium (Ca+) is taken out of solution by
biological organisms for the building of shells and skeletons
or by chemical precipitation, which leaves sodium to constitute
the lion's share of the four common salt cations in ocean waters.
Water has a large dipole moment, a property of its
molecular structure that enables it to dissolve almost anything.
The dipole moment arises because oxygen is more negatively charged
than hydrogen; thus, oxygen pulls in the shared electrons,
increasing the electron density around itself.
The greater the amount of mean annual precipitation, the greater the amount of leaching of the underlying soils.
Therefore, subhumid to superhumid climates find their soils devoid of nutrients.
The smaller the amount of mean annual precipitation, the lesser the possibility
of leaching of the underlying soils. Therefore, semiarid to superarid climates
find their soils filled with nutrients.
Exorheic, or peripheral drainage basins carry the surface waters from headwaters to ocean, delivering their load of salts and closing the hydrologic cycle. Conversely, endorheic, or Lakes in arid regions tend to have high salinity,
while lakes in humid regions display an opposite trend.
The extra salt present in a given soil profile, to be mobilized by subsurface runoff into local and regional drainage, may originate in one of the following sources: (1) old natural salts; (2) new natural salts; (3) old artificial salts; and (4) new artificial salts.
The irrigation of arid lands effectively creates
new salts
by partitioning the relatively young soils to extract the useful salts
(magnesium and potassium), while disposing of the waste salts (sodium
and calcium), the latter to be removed by appropriate drainage.
As a stream or river flows from headwaters to ocean,
its increase in salinity due to the accumulation of evaporation has a distinct natural flavor.
Not all irrigation projects produce
a comparable amount of waste salt. The quantity of waste salts tends to be much greater
in arid regions than in humid regions.
The salt disposal problem may be somewhat
manageable when the irrigation project site is reasonably close
to the nearest ocean, say, along the coast, or within a short distance from it.
10. OUTLOOK
Irrigation is shown to be
a mixed bag. On one hand, the irrigation
of arid lands may result in a very productive enterprise; on the other hand, it
is very likely that the increase in productivity will be coupled
with a comparable increase in the amount of waste salts, which would need to be disposed
of properly, usually at great expense. Barring effective disposal of salt waste,
the local environment will be saddled with new salts
and these will invariably result in the degradation of the landscape.
It is proposed here that societies must
learn to pay for the adequate disposal of waste salts produced by irrigation.
REFERENCES
American Society of Civil Engineers. 2012. Agricultural Salinity Assessment and Management.
ASCE Manual and Reports
on Engineering Practice, Manual 71, Second Edition.
L'vovich, M. I. (1979). World water resources and their future. Translation of the original Russian edition (1974), American Geophysical Union, Washington, D.C.
Pillsbury, A. F. 1981.
The salinity of rivers.
Scientific American, Vol. 245, No. 1, July, 54-65. Extract.
Ponce, V. M. 1995.
Hydrologic and environmental impact of the Parana-Paraguay waterway on the Pantanal of Mato Grosso, Brazil.
https://ponce.sdsu.edu/hydrologic_and_environmental_impact_of_the_parana_paraguay_waterway.html
Ponce, V. M. 2005.
The Salton Sea: An Assessment. Online article.
Ponce, V. M. 2007.
The facts about San Joaquin valley drainage. Online article.
Ponce, V. M. 2015.
Groundwater utilization and sustainability. Online article.
Ponce, V. M. 2015.
The right of Nature to dispose of its
salt waste. Online article.
Ponce, V. M. 2019.
The properties of water.
Online article. https://ponce.sdsu.edu/properties_of_water.html
Ponce, V. M. 2023.
The drought toolbox.
Online article. https://ponce.sdsu.edu/drought_toolbox.html
Rhoades, J. D., D. B. Krueger, and M. J. Reed, 1968.
The effect of soil-mineral weathering on the sodium hazard of irrigation waters.
Soil Science Society of America Proceedings, Vol. 32, 643-647.
|
| 230915 |